History
SYNTHESIA, a. s. is a major European manufacturer active in the area of special chemicals, backed up by 100 years of history. It manufactures hundreds of products for customers in 70 countries all over the world. Synthesia employs around 1400 workers, its total annual sales in 2023 achieved approximately 188 million EUR.
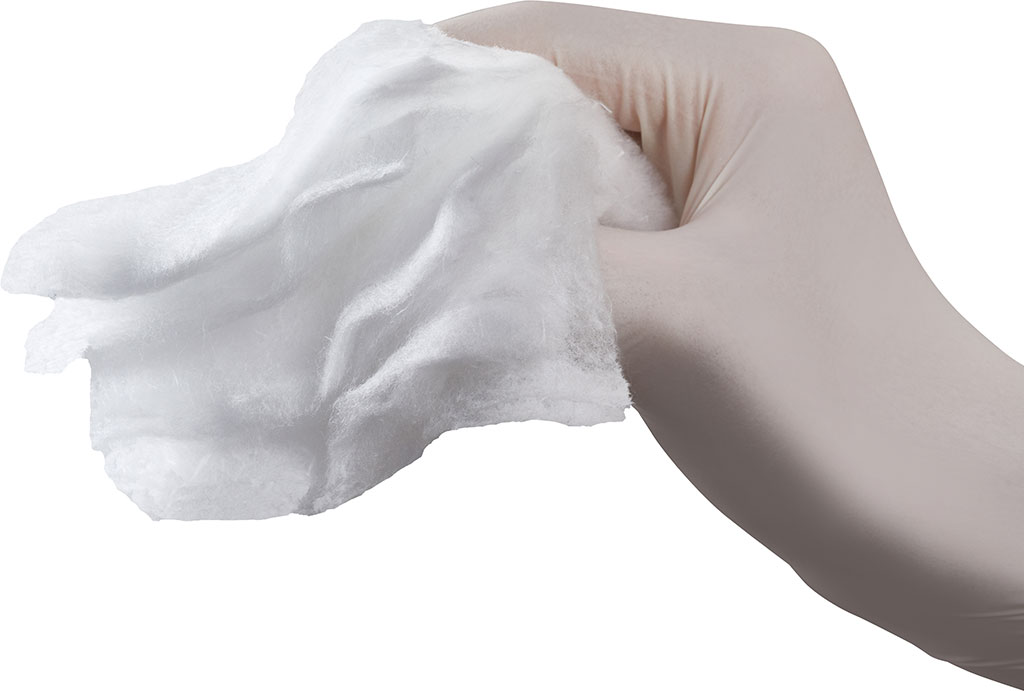
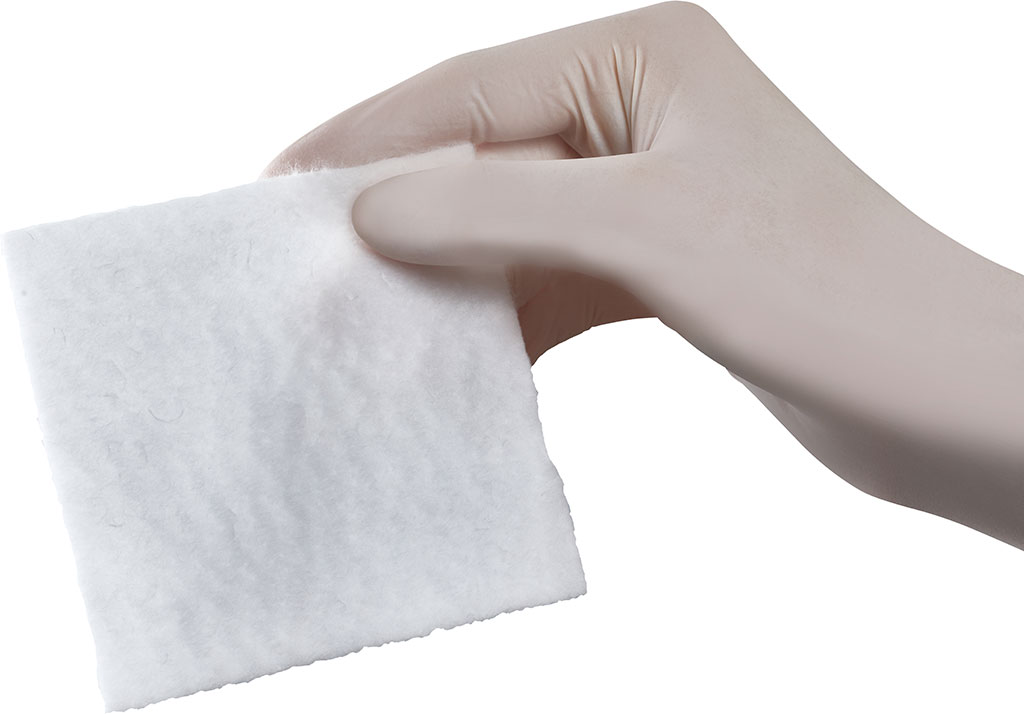
From the medical point of view, Synthesia is an original Czech manufacturer of oxidized cellulose based haemostatic products, marketed under the registered trademark OKCEL®, which are used in biomedical segment with particular focus on professional surgery. OKCEL® product range is manufactured within the Business Unit Oxycellulose, which is a part of SBU Nitrocellulose, one of the strategic business units of Synthesia, a.s.
-
1920
the history of our company begins with foundation of “Československá akciová továrna for explosive chemicals”
-
1923
start of nitrocellulose production (energetic grades)
-
1925
start of production of nitric and sulphuric acid
-
1939
start of plant construction for the production of dyes and pharmaceuticals in Pardubice - Rybitví
-
1942
start of API (Active Pharmaceutical Ingredients) production
-
1950
start of nitrocellulose production (industrial grades)
-
1994
Synthesia is transformed into a joint stock company
-
90´s
gradual development of oxidized cellulose production
-
2004
OKCEL® trademark assignment
-
2006
new plant for production of oxidized cellulose and its derivatives
-
2009
the only majority shareholder becomes AGROFERT HOLDING, a.s
-
2010
assingment of CE mark for haemostatic products marketed under the brand OKCEL®
launch of products OKCEL® H-T (standard knitted form) and OKCEL® H-D (heavy duty knitted form) -
2012
introduction of a non-woven absorbable haemostat OKCEL® F with layered structure
-
2020
OKCEL® S introduction - new innovative strengthened cotton wool form of oxidized cellulose based haemostat
-
2024
the KAPRAIN Group becomes the only majority shareholder
Quality and manufacturing
OKCEL® haemostats are classified as a class III medical device. They are CE marked by The Institute for testing and Certification, Inc., that confirmed compliance with all relevant EU standards regulating the production of medical devices.
Our quality management system has been designed to maximize overall efficiency throughout the process chain as well as customer satisfaction and meet requirements of the international standard ISO 13485: 2016 and the European Medical Device Regulation 2017/745 (MDR). Our company has been certified by The Institute for testing and Certification, Inc. to ISO 13485:2016 Quality Management System Standard and by Bureau Veritas Certification Holding to ISO 9001:2015, ISO 14001:2015 and ISO 45001:2018. Besides, Synthesia belongs to founder members of a programme called “RESPONSIBLE CARE”. After its successful tenth defense in August 2022, we acquired the right to use this logo until October 2027.
We put emphasis on the trained production staff, our employees keep in their mind that OKCEL® products, they manufacture, have been designed for human use and must be thus safe and effective. At all levels, we strive to simplify surgical procedures while improving patient care.
years of experience with haemostats
millions of surgical procedures
world continents
The BU Oxycellulose quality policy:
TOP management undertakes to manufacture safe and fully functional medical devices in accordance with applicable legal / regulatory requirements and to adhere to the surveillance and vigilance procedures relating to medical devices in order to ensure customer and end user satisfaction.
To achieve this goal, we have a team of skilled and competent staff, where each manager is responsible for enhancing quality in their department, while taking into account the quality improvement objectives not only in their area but throughout the company. Thus, each worker contributes to realizing the company's strategic goals.
In addition, TOP management undertakes to maintain the effectiveness of the quality management system using a process-based approach to setting goals for each quality management system area that is annually reviewed.
Sustainability
Environmental, social and governance (ESG) responsibility towards our employees, customers and society as a whole is firmly embedded in our corporate strategy. Our commitment to sustainability towards current and future generations is a natural part of all our company's activities, it is reflected in corporate guidelines, but also in development projects and long-term activities.
Improvements over the last 5 years:

|

|

|
||
| Thanks to the investment in greening the energy source for 1.75 billion CZK, we reduced CO2 emissions by 60 % compared to 1990. | In addition to CO2 emissions, energy consumption and heat production from fossil fuels also decreased during the monitored period. We are gradually increasing the ratio of using fuels from renewable sources instead of fossil fuels. | In addition to the saving of water used, there was also a significiant reduction in the volume of wastewater discharge, the pollution of which was bellow the level of charging limits. |
Synthesia's global responsibility is clearly documented in the Sustainable Development Report.
Cooperation
We are aware that research and development is a key area for further company progress. Annually, we invest higher and higher financial resources from net sales back into research projects, facilities and qualified human resources. We understand R&D as something that pushes us forward and creates a daily edge over our competition.
In order to maintain a high standard of product quality and end-users satisfaction in the introduction of new product innovations, Synthesia, a.s. cooperates closely with educational institutions in the chemical and technical fields, research institutes, surgeons and other medical professionals, including the following:
Our cooperation with educational institutions takes many forms, including excursions, apprenticeships and assisting students in preparing their expert theses. We offer a TRAINEE programme designed specifically for university graduates.